With this recent publication, a spotlight has once again been shone on the fact that the public has long perceived the pharmaceutical industry as putting profits before patients, and in turn, not being fully transparent with their data. The fire of this perception was fed in part by the first iteration of the GPS, published in 2015, which did not paint a rosy picture of Big Pharma’s compliance with clinical trial transparency regulations.
Bioethics International conducted an analysis of all clinical trials involving drugs approved by the Food and Drug Administration (FDA) in 2014 that were sponsored by large pharmaceutical companies. Companies were rated on their drug-level compliance with clinical trial registration, results reporting, clinical study report (CSR) synopsis sharing, biomedical journal publication, and the regulations put forth in the FDA Amendments Acts (FDAAA).
In 2014, the FDA approved 19 new drugs for which 11 large companies were the Sponsor. A total of 533 trials supported the new drug applications (NDAs) submitted by these sponsor companies. Analysis of 505 of these relevant trials was conducted, and the following items were assessed:
- Number of trials registered in any public registry, including corporate and international registries
- Number of trials for which results or a CSR synopsis was provided in the registry
- Number of trials published in an appropriately indexed journal
- Number of trials subject to FDAAA that met that statute’s transparency requirements and the extent to which these requirements were met
Results from the 2014 analysis are encouraging, and they point to the take-home message of increased compliance with transparency regulations across the board.
Jennifer E. Miller, Ph.D., founder of Bioethics International and lead author of the paper publishing the results of the 2017 Scorecard, said to Outsourcing-Pharma “This year’s Scorecard shows clear corporate leaders in clinical trial transparency and industry improvement on several metrics. We hope this improvement continues year after year, because clinical trial transparency is critical for advancing innovation, respect for trial participants, and patient health.”
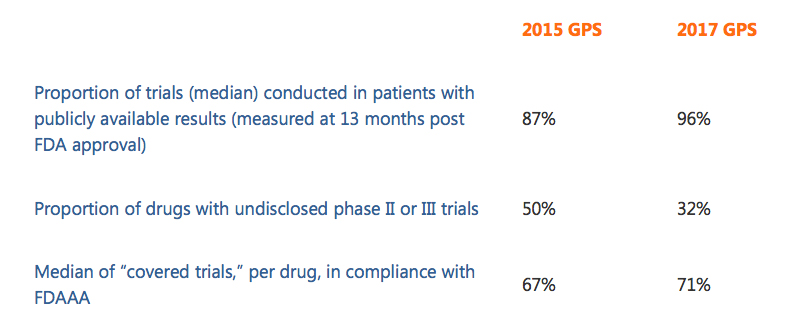
The 2017 Scorecard reports that, per drug, a median of 100% of trials in patients were registered in a public registry, and that 71% of these registered trials reported results in a registry or shared a CSR synopsis. A total of 96% of trials achieved the benchmark of making results publicly available in some form by 13 months post FDA approval. These results become even more meaningful when compared to results from the 2015 analysis:
Authors of another similar study recently published in the peer-reviewed journal Current Medical Research and Opinion (CMRO), conducted an analysis of the compliance rate for the disclosure of results for company-sponsored clinical trials involving new medicines approved by the European Medicines Agency (EMA) during 2014.
The study primarily measured the percentage of trials for which results had been disclosed on a registry or in the scientific literature either (a) within 12 months of the later of either first regulatory approval or trial completion, or (b) by 31 July 2016 (end of survey). Of the 542 trials conducted with 32 newly approved drugs that were sponsored by 22 companies, 505 (93%) had results that had been disclosed within 12 months, and 518 (96%) had results that were disclosed by 31 July 2016.
This same analysis was conducted on new drugs approved in Europe in 2009, 2010/2011, and in 2012/2013:
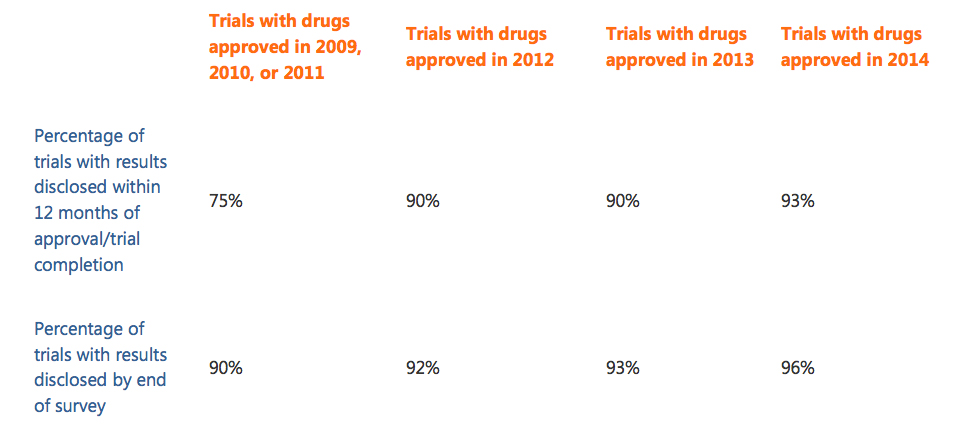
As with the BMJ Open study, a clear upward trend was seen in compliance for trials conducted using drugs approved in Europe. Given that sponsor companies operate in a global drug development market, it’s encouraging to see that increased transparency is not an initiative that is unique to the U.S.
These results, however, should be taken with a grain of salt. The GPS only tells the story of Big Pharma, comprised of companies with a lot of resources and much riding on public perception. The Sponsor companies that were “scored” in this analysis typically have a team in place – Clinical Operations, Legal, Regulatory, Disclosures/Transparency – that have a solid understanding of the regulations driving transparency and the resources and support that are needed to implement a plan for adhering to these regulations. Smaller biotech companies and academic institutions are often not so fortunately positioned, and as a result, have not been able to make the commitment to increased transparency that Big Pharma has.
Nevertheless, the increase we’ve seen in transparency compliance can be attributed to the fact that companies are hearing the public’s concern that they are not putting the proper value on patients, and to groups like Bioethics International that keep pushing industry leaders to share more and to share it in a patient-friendly format.
The call for increased transparency is growing louder, and further progress toward the goal of sharing more, not less will be encouraged by analyses planned for the future, including a data sharing analysis in the 2018 Scorecard.
References:
Fassbender, Melissa. “What Makes a ‘Good’ Pharma Company? J&J, Sanofi Lead Clinical Trial Transparency.” Outsourcing-Pharma.com, 19 Dec. 2017.