CLINICAL TRIAL REGISTRATION AND RESULTS DISCLOSURES Services
Industry experts with hands-on experience working with regulatory agencies
Our teams are here to advise, prepare, automate and manage compliance with your disclosure obligations.
MMS helps Sponsors achieve and surpass clinical trial disclosures reporting requirements for clinicaltrials.gov, the European Union Clinical Trials Register (EU-CTR), EU Clinical Trial Information System (EU-CTIS), and other international registries. Our robust services, processes, and tools allow MMS to be your centralized transparency department with the ability to manage all workflow and clinical data reporting obligations for an entire pipeline.
As disclosure legislation has evolved multi-fold across the world over the past two decades, MMS Clinical Trial Disclosure experts are here to advise, prepare, automate, and manage compliance with Sponsors’ disclosure obligations.
MMS offers:
- Simple workflows to progress studies through the disclosure lifecycle
- Insights into transparency requirements for all global registries
- The ability to share data with multiple registries from a single data set
- Evaluate of compliance risks and identify bottlenecks in operational processes
- Best practices based on our industry experiences and knowledge of Sponsor’s SOPs and organizational structure
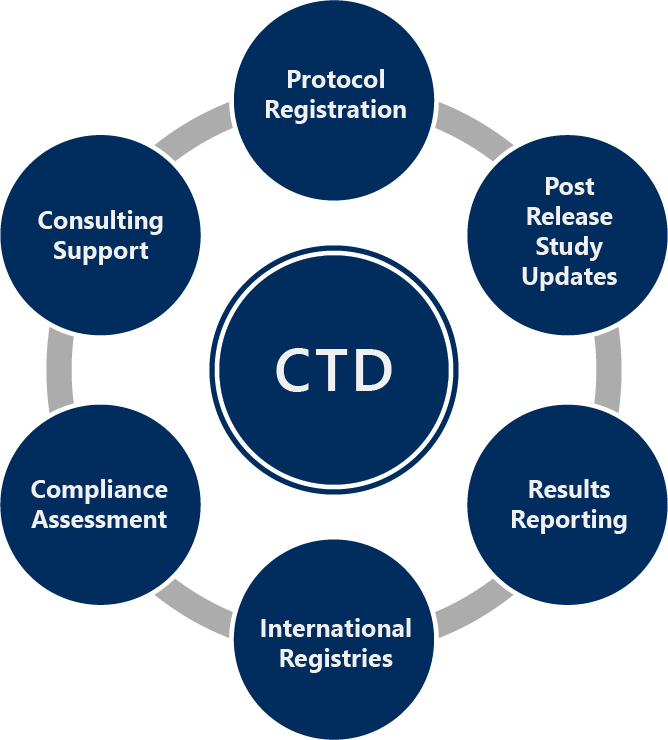
MMS Provides:
- Protocol registration to clinicaltrials.gov, EudraCT, EU-CTIS, and more
- Post release site and status updates, protocol amendment updates in accordance with the registry and regulatory requirements
- Results posting to clinicaltrials.gov, EudraCT, EU-CTR, and several local registries
- International registries support
- Redaction of protocol, statistical analysis plan, and synopsis for clinicaltrials.gov and company websites
- Compliance assessment
- Consulting support through training and workshops
Why MMS for Clinical Trial Disclosures Services:
- Quality-focused services: 98% of US registration accepted by the National Institute of Health (NIH) in first release)
- Industry experts with combined experience of over 55+ years in the clinical trial disclosure space
- Seamless integration with client teams for workflow management of disclosure deliverables
- Excellent domain knowledge, including registry databases, therapeutic areas, review criteria of regulatory agencies, and more.
- Hands-on experience of working with regulatory agencies like the US-NIH and the EMA
The MMS Difference
The clinical trial disclosure services within our transparency organization is dedicated to delivering high-quality, timely, and compliant clinical trial information and data for patients, researchers, and the general public.
The MMS Clinical Trial Disclosure team aspires to add value to our Sponsors, trial participants, and the general public through responsible sharing of clinical trial information and data. We work tirelessly to mitigate risk to our Sponsors and ensure the privacy of patients. We strive to be the partner of choice for Sponsors and be recognized as an industry leader in the clinical trial disclosure domain.
Learn More With These Insights
Blog
How to Train Users on the Clinical Trial Information System (CTIS) Ahead of New Regulations
Get in touch



