How to Avoid a Form 483s in your Analytical Laboratory
Depending on the circumstances and the items reviewed, you may be unable to avoid receiving a Form 483 at the close of your inspection. However, you can implement five simple processes from our experts in your laboratory to help reduce your chances of getting one of those feared and dreaded Form 483s at the close of your next FDA inspection.
(1) A Daily Laboratory Monitoring Program
You have probably heard the saying, “you don’t get a second chance to make a first impression.” This is even true when the FDA pays your laboratory a visit. Walking into a neat and orderly space gives the inspectors the impression that you must have good procedures and care about your work.
Since the audit tour is typically very early in the inspection, if an audit tour goes badly, it can set the inspectors up to expect a less-than-favorable visit.
Implementing a daily laboratory monitoring program can ensure that the laboratory space is neat and orderly for an unexpected visit by the FDA. Performing a daily inspection of the laboratory space, typically by a quality assurance employee, can allow time to correct items found in the laboratory that may trigger an inspector to issue an observation.
Reducing clutter, ensuring expired materials have been removed from the working space, and checking for proper and consistent labeling practices are among a few items that can be reviewed during daily QA inspections.
Daily inspections also set the expectation for all employees that order, cleanliness, and company procedures should always be followed and not only during times of inspection.
(2) Data Review Process
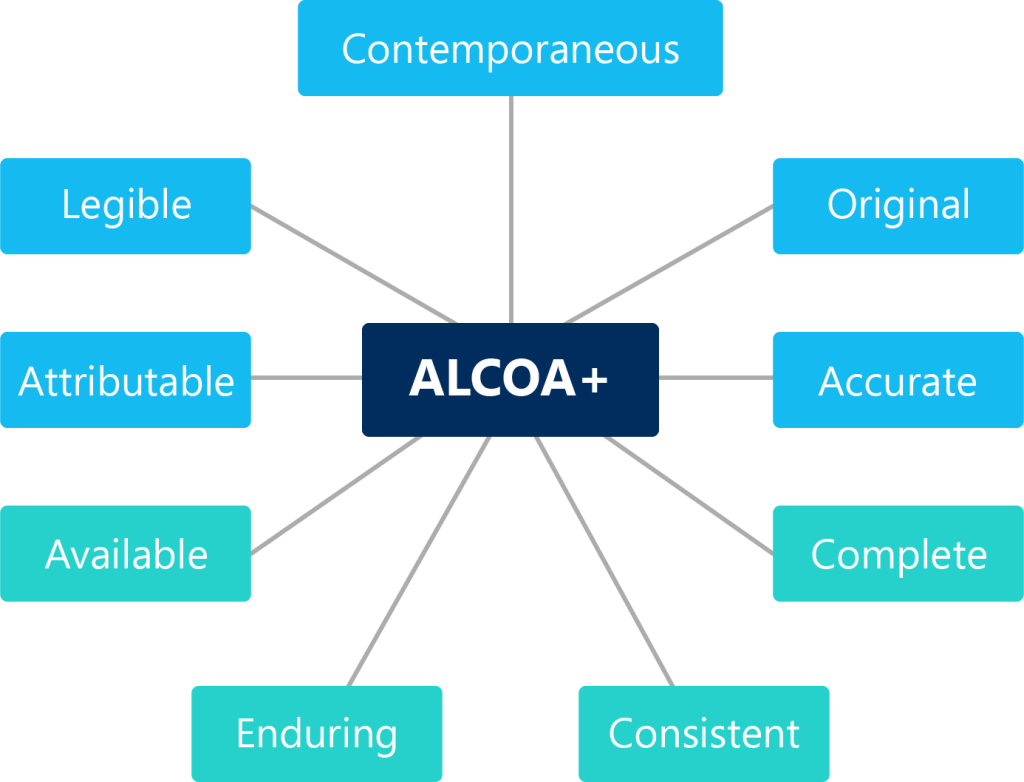
When reviewing data, ensure it follows the ALCOA + principles:
- (A)ttributable: identifiable to the person or system that generated the data
- (L)egible: data can be read and understood
- (C)ontemporaneous: recording data when it occurs
- (O)riginal: original recording of data should be the main record; not copies or transcriptions
- (A)ccurate: a reflection of what happened; error-free
+
- Available: accessible
- Enduring: ensuring data is available long-term
- Consistent: chronological and in an expected sequence
- Complete: nothing has been deleted or lost
(3) Internal Audit Program
Internal audits are performed to assure compliance to all applicable requirements and to discover areas that may need improvement. The audits should be performed by a qualified auditor that is unrelated to the activity being audited. Some areas that should be considered for internal auditing are:
- Data Records
- Equipment Records
- Electronic Systems
- Investigations/Deviations
- Quality Department
(4) Continuous Improvement Plan
Areas of improvement may be discovered during daily laboratory monitoring or internal audits. Often, reducing the frequency of issues can be achieved through SOP revisions, implementation of new processes or forms, or additional training provided to employees.
(5) Effectiveness Checks
Once improvement initiatives have been completed, there must be a process for determining if the actions actually resolved the recurring issue. If not, a new improvement plan may be needed. Effectiveness check processes should include a defined timeline for checking the effectiveness, parameters to evaluate the success, and a plan for those identified as not successful.
Considerations for Implementation of New Processes
It’s not always easy to implement new processes in organizations; there are things to consider. The resources, time, and cost of a new process must be evaluated prior to creating and launching them. Also, changes may not come easy for some employees, and you may be met with resistance. Getting buy-in from upper management and those affected by the new processes is important.
If any of these processes are missing from your laboratory, reaching out to the MMS Quality & Compliance Team is a great first step! Our experienced and knowledgeable team can help you implement these processes. Showing continual improvement of processes may help you avoid one of those ever so unpopular and dreaded 483s. Reach out to our team at info@mmsholdings.com.





