Quality and Compliance services
Expert support to meet the demands of the ever-shifting global regulatory environment.
Our proven expertise, knowledge, and processes give sponsors flexible solutions to meet rigid compliance requirements.
GxP Audit Services
We recognize the importance of implementing fit-for-purpose policies, procedures, personnel training, vendor management, vendor oversight, and auditing activities. We also recognize that compliance with regulatory requirements is especially difficult in this era of global footprints and telework. To support our clients, we have a full suite of Auditing and Compliance Services, including the capability to perform audits remotely, in-person, or via a hybrid combination of these approaches as required by on-the-ground activities and auditee availability.
Why MMS for Auditing?
When you choose MMS Compliance & Auditing Services, Industry experts backed by strong processes will be assigned to your projects.
- The MMS team is known for our well-defined processes and relentless commitment to delivering the highest quality results on time consistently.
- We assign each sponsor a dedicated point of contact that manages the delivery of audit work to hit timelines, ensuring consistently high-quality deliverables and transparency of KPI reporting.
- Best-in-class reports are provided in a third of the time as the industry standard: 10 business days for standard audits and 45 calendar days for mock inspection peer-reviewed report delivery.
We have GxP audit compliance services specialists located in the US, Europe, the UK, Latin America, Asia-Pacific, and Africa available for global assignments.
Types of Audits Across GxP Environments
- Investigator Site Audits
- Routine, Targeted and For-Cause
- Inspection Preparation/Training
- Phases I-IV
- Mock Inspections/Pre-Approval Inspections
- Vendor Audits
- Routine, Targeted, For-Cause
- Qualification/Re-Qualification
- Sponsor Internal, Process, Oversight, and Gap Assessments
- CRO
- Project Management
- Monitoring and Site Management
- Data Management
- Statistical Programming/Biostatistics
- Medical Writing
- Clinical Trial Supply/Distributors/Depots
- Interactive Response Technology
- Translation Services
- Archive Facilities
- Medical Monitoring
- Centralized Imaging
- Central, Bioanalytical and/or Specialty labs
- Drug Substance/Biologics Manufacturers
- Document Audits
- Case Report Forms
- Informed Consent
- Investigator Brochures
- Clinical Study Reports
- Development Safety Update Reports
- Protocol Audits (all Phases)
- Trial Master File Audits (paper/electronic)
- System Audits
- Bespoke audits
- Sponsor System/process audits
- Clinical Database audits
- Pharmacovigilance systems
Inspection Readiness and Preparations
Give your organization the ability to respond confidently to regulatory authority inspections or investigation requests with inspection readiness and preparations by MMS. Led by former health authority personnel, MMS experts provide comprehensive pre-inspection reviews of facilities, processes, documents, and staff member preparedness.
MMS conducts mock regulatory authority inspection audits to ensure that all sponsor staff is prepared for the variety and volume of requests accompanying any regulatory inspection at the plant or facility, third-party vendors, suppliers, laboratories, clinical sites, and/or contract manufacturers. Additionally, process, document and facility audits are conducted in conjunction, providing the added benefit of uncovering potential observations and allowing for resolution or mitigation before the actual regulatory authority inspection.
Uncovering and addressing findings at this primary juncture provides sponsors the added security of knowing that all inspection and compliance potential areas are under control.
Related Resources
Quality assurance is vital in outsourcing to meet regulatory and quality requirements. Read more about incorporating QA in outsourcing can reduce business risk, time and effort.
Quality Assurance Services
Quality is critical in all phases of pharmaceutical development and becomes a strict requirement as the product moves to a marketed product. MMS provides experienced quality experts where and when you need support for building, auditing, improving, or defending your Quality Management Systems.
Why MMS for Your Quality Needs?
When you choose MMS for your compliance services, you’ll work with experienced quality professionals backed by strong processes.
- Quality resources are necessary but can be costly for a full-time employee. MMS can provide years of experience with multiple areas of expertise in quality and align with how much or little time you need for your QMS.
- We assign each sponsor a dedicated point of contact with at least 10 years of experience working within quality. Many MMS employees are previous QA Managers or Directors.
- Your point of contact manages the deliverable to meet timelines, ensuring consistently high quality of deliverables and transparency of KPI reporting.
- The MMS team is known for our well-defined processes with robust internal systems support and our relentless commitment to consistently deliver the highest quality results on time.
- We have GxP compliance service consultants located in Europe and the US available for global assignments.
Quality as a Service
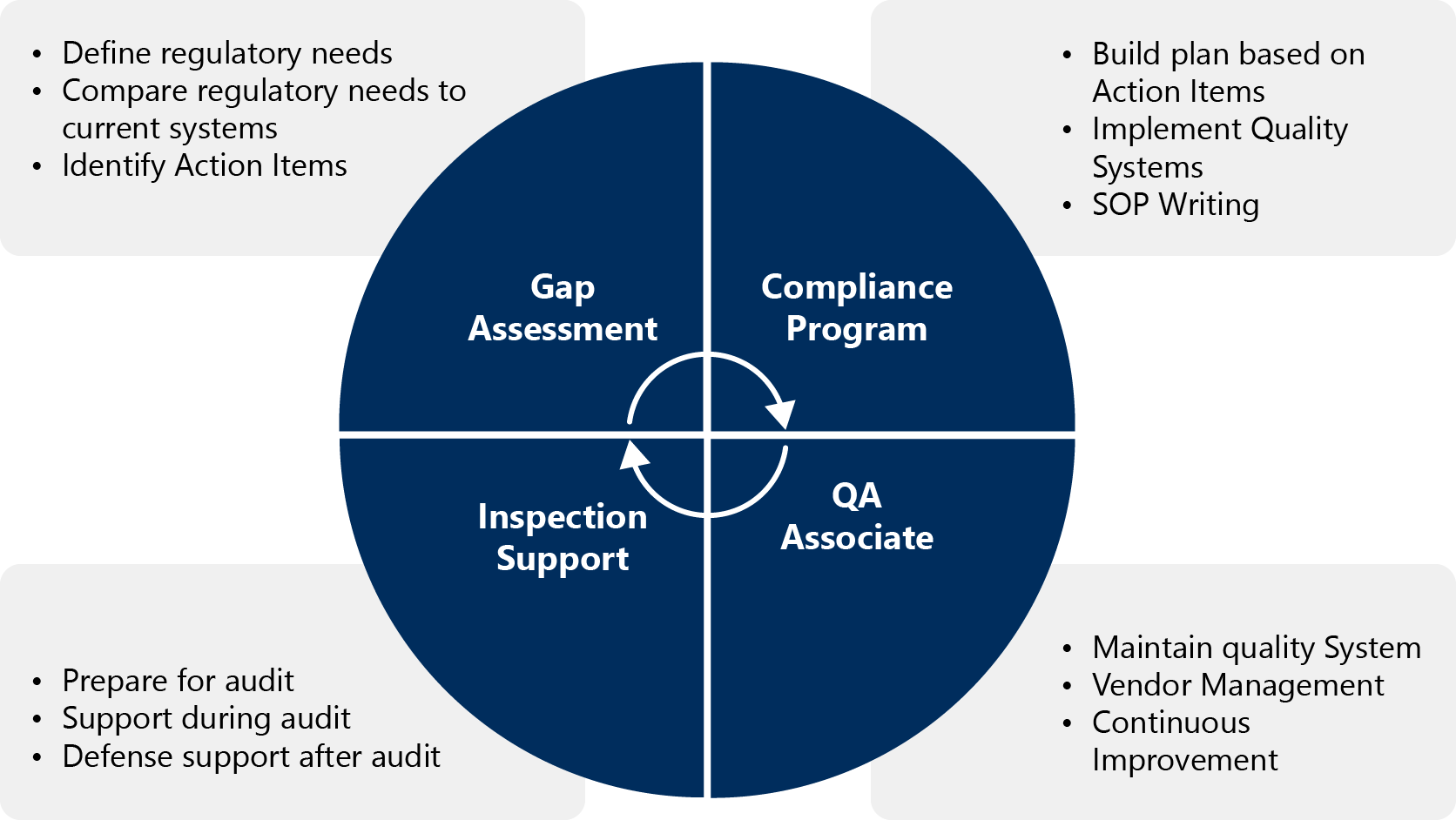
Quality Compliance
Uniquely focused on quality and compliance, MMS supports a wide range of sponsors in need of Quality Management Systems (QMS) development, including those completing large-scale mergers and acquisitions that may need to streamline and improve an existing QMS. MMS experts can provide the support to develop systems & tools for all quality & compliance processes.
At MMS, there is no one size fits all approach to providing QMS services – as all organizations have inherent differences. This forward-thinking, risk-based approach ensures that sponsors gain efficiency and reduce the risk of non-compliance with customized programs for:
- QMS Evaluation and Gap Assessment. Understand the strengths and weaknesses of your current quality management system.
- Procedure Support, including a thorough evaluation and customized QMS project plan that ensures end-to-end compliance through policies, process maps, standard operating procedures (SOPs), work instructions, and associated forms and templates.
- Organizational Structure Support, including implementing organizational charts, position descriptions, development of strategic business planning framework, and key performance indicators.
Quality Assurance Associate Program
Resourcing for Quality Assurance can be time-consuming and can be costly. Without the right person filling the role, the cost can come at the price of compliance. MMS can provide the resources to support your quality systems to supplement the need for a full-time employee.
Quality Systems MMS Experts Can Support:
- Organization and Personnel (job descriptions/training)
- Training Coordination
- Vendor Management
- Document Control
- Data Integrity
- Record Management
- Change Control
- Validation Plans
- Management Review/Quality Metrics
- Label Control
MMS has Experts in Compliance Oversight:
- Facility Maintenance and PM program
- Equipment Maintenance and PM program
- Production and Process controls
- Laboratory / Quality Control requirements
- Internal Audit Program
- Errors and Corrections
- Continuous improvement
- Complaints
Vendor Management
Ultimate regulatory obligations to quality requirements are the sponsor’s responsibility, regardless of whether performing in-house or through a contracted vendor.
CRO and CMO partnerships are important throughout the drug development lifecycle, but the responsibility does not end with creating and fulfilling the contract alone.
Sponsors must qualify, evaluate, and assess their vendor partners on an ongoing basis and maintain adequate records to prove these activities were completed. In recent years, there has been a shift to a heavier focus on risk-based monitoring, implementation of quality agreements, and use of quality indicators.
MMS offers a full suite of vendor management services as well as ad hoc vendor management support to meet regulatory requirements for vendor oversight.
Our vendor management services include:
- Development of vendor management program procedures, KPIs, and KQIs to fit your company’s needs.
- Gap assessment of a current vendor management program to determine the areas of concern.
- Create Quality Agreements, supplier evaluation questionnaires, and supplier surveillance questionnaires.
- Vendor qualification or requalification.
- Routine and for-cause audits.
MMS has the proven expertise, knowledge, and processes to provide organizations with regulatory-compliant Vendor Management Services or create a complete custom program.
Training Coordination
Training in a compliance setting can be complex. You have to be able to show that you have people qualified for the position assigned and that they understand the quality responsibilities of their job. Once qualified, you must continually educate and provide proof of continual proficiency.
A well-thought-out and managed training program is the pillar of a quality program. Training encompasses not just the job to be done but an understanding of the regulations/accreditations and the quality policies established.
MMS has experienced employees to serve as a training partner in your GxP Training needs:
- Perform a gap assessment of the current training program to evaluate training needs, training requirements, the effectiveness of the training, and the documentation needed.
- Create an audit-ready training program that will provide evidence that you meet and exceed quality requirements for qualifying employees and maintaining proficiency.
- Develop Training Standard Operating Procedures and training documentation.
- Create training modules or courses,
- Create GxP training or Inspection Readiness courses to be presented by our staff or yours.
- Implement an electronic Learning Management System (LMS) to track all training requirements and completed training in one location.
Inspection Support
Due to a shift in qualifying contract services through audits, the amount of time spent hosting audits has significantly increased. MMS can provide the resources to manage your hosted audit program.
More importantly, MMS has experienced employees that have hosted regulatory and accreditation body audits. We will partner with you through our quality programs to build, improve, and defend your quality management systems.
MMS inspectional support can include:
- Assist in preparation for a scheduled or unscheduled audit (inspection readiness)
- Provide comprehensive pre-inspection reviews of facilities, processes, documents, and staff member preparedness
- Train employees on hosting audit
- Create inspection procedures and documentation
Regulatory Defense
FDA 483 observations and warning letters are openly available and can cause concern to the public or be used by competitors as an advantage over you. Addressing the issues swiftly and sufficiently is extremely important and is best handled by experts in the quality system is addressed. MMS can provide the resources to:
- Evaluate the finding, investigate root cause and identify risks
- Prepare a corrective action plan
- Draft response to the finding
Get in touch

