With all new regulations like Health Canada-PRCI, EMA Policy 0070 and EU-CTR requiring the publication of clinical information, it’s important to understand what is considered company confidential information (CCI) and how to protect the information being shared with the public. In this blog, we will go over what CCI is and how to identify and handle it.
What is CCI?
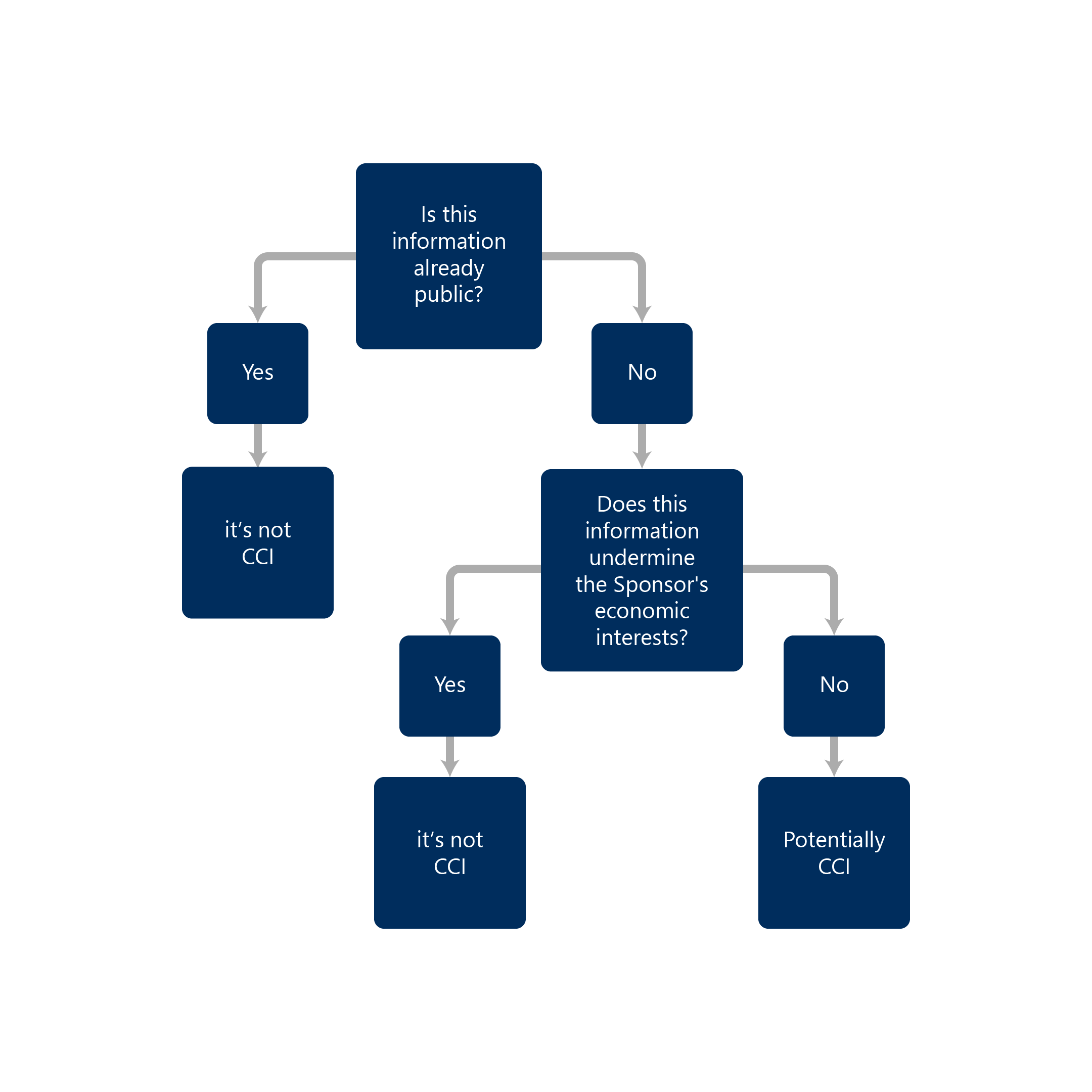
CCI is considered any company confidential information that could cause significant damage to commercial interest if disclosed.
Criteria include information that:
- Is not publicly available
- A person has taken reasonable measures to ensure it remains not publicly available
- Has actual or potential economic value to the person or their competitors because it is not publicly available, and its disclosure would result in material and financial loss to the person or a material or financial gain to their competitors
How to Conduct a Literature Search for Potential CCI?
Prepare a list of key terms for a targeted literature search. The search will be based on different permutations and combinations of the identified terms.
Once these terms are ready, we can begin our search to check if the information is already in the public domain.
If you find any of this information during our search, the information is not considered CCI.
If the information is not publicly available, the information can be a potential CCI depending on fulfilling the above-stated criteria.
For all proposed CCI redactions, the Sponsor should submit a specific, pertinent, relevant, not overstated, and appropriate justification for each piece of the text proposed for redaction. Justification tools are used as communication between the regulatory agency and the Sponsor during the redaction consultation process.
The robust justifications should clearly identify the information to be redacted, highlight how this information will be taken in the context of public knowledge, explicitly indicate its relevance to ongoing development programs, and explain the economic interest or competitive position for redacting the information.
Why did the CCI justification get rejected?
Most of the clinical information provided in clinical reports will not consist of CCI. However, there are many reasons why the regulatory agency would reject CCI redaction requests.
The most common reason for rejection by a regulatory agency is the presence of the proposed information in the public domain during a literature search. In addition, the regulatory agency can reject proposed information due to a lack of innovative features or the disclosure of the information is in the public’s interest.
The agency can also reject redaction if the justification provided is insufficient or irrelevant.
There are circumstances where CCI gets accepted. The regulatory agency decides what should be considered CCI and will accept or reject your justification for redaction.
By: Midhun Madham, Associate Manager of Clinical Trial Transparency, and Manish Yadav, Clinical Trial Transparency Team Lead
If you want more information about CCI or redaction, please email info@mmsholdings.com or visit our website.
