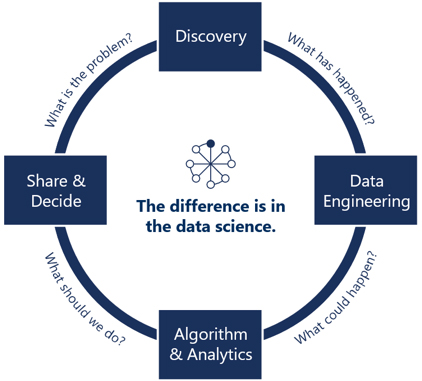
Security and auditing: Data landing, storage, cleaning, standardization, analysis, prediction, and security
Authored by MMS experts Chris Hurley, Aditya Gadiko, Srinivas Mukkala, and Kulin Mehta, the paper’s abstract is as follows:
Who could have imagined that a few simple ASCII datasets would pose such a huge “big data” challenge? Therein lies the paradox, while the data is simple and there are relatively few variables to analyze, the volume is huge and presents a great processing challenge. This poster demonstrates a repeatable “big data” solution that utilizes FAERS data, provided quarterly by the FDA, to provide the answers to post-marketing safety surveillance questions. The process, techniques, and tools used to handle and analyze such massive amounts of data, in a clinical setting, are demonstrated here, creatively enabling us to cut the Gordian knot and overcome a seemingly impossible challenge.
To learn the robust clinical data science process behind this abstract, read the full paper here.