It would be relatively easy if biopharmaceutical manufacturers could develop a perfect process one time and then rely on it to deliver the same high-quality products to all patients in all future scenarios; yet, changes in processes and sites are often necessary to meet the dynamic and challenging landscape of monoclonal antibody manufacturing throughout the drug development lifecycle.
Required changes may stem from improvements in process efficiencies, raw material changes, supply chain issues, evolving regulatory requirements, increasing production to meet patient needs, or unforeseen circumstances. No matter how the changes come about, comparability studies for biologics are key to demonstrating that the post-change product maintains the same safe, effective, and high-quality attributes as the pre-change product (ICH, Q5E, 2004). Ultimately, for regulatory submissions, it is the strength of the comparability data that enables manufacturers to carry on with the day-to-day operations necessary to support patients.
What are comparability studies for biologics and why are they necessary?
For complex biologics, even seemingly small changes, like attempts to increase yield through small cell culture tweaks, can greatly impact product quality down the line. These differences may not always be apparent until the molecule is pressure-tested through rigorous head-to-head extended characterization and/or forced degradation studies.
Per the ICH Q5E guideline, demonstrating “comparability” does not require the pre- and post-change materials to be identical, but they must be highly similar. The guideline requires that, “…the existing knowledge is sufficiently predictive to ensure that any differences in quality attributes have no adverse impact upon safety or efficacy of the drug product”.
The overall intention of the comparability package is to provide regulatory authorities with a transparent pathway from the safety, efficacy, and quality data from pre-change clinical batches to post-change batches based on a strong foundation of science and thorough understanding of the highly similar, and oftentimes improved, product.
Depending on the phase of development, a complete comparability package for the drug substance may comprise several studies, including:
- extended characterization,
- forced degradation,
- real-time and accelerated stability studies, and
- statistical analysis of the historical release data.
While release testing and stability studies in biologics are routine, planning for extended characterization and forced degradation studies should take place well in advance of major manufacturing changes so that test methods, critical quality attributes, and inherent molecular characteristics are well understood before facing the level of scrutiny that the complex comparability study will undergo.
With the guidance from cross-functional teams, including Regulatory and Purification SMEs, the CMC team should determine when a comparability study for biologics is warranted, keeping in mind the proposed change, potential risks, phase of development, and planned regulatory filing. Besides release and stability testing, it is advisable to begin extended characterization and forced degradation studies early in development. An example strategy is shown in Table 1 below.
Table 1: Example of phase-appropriate comparability testing
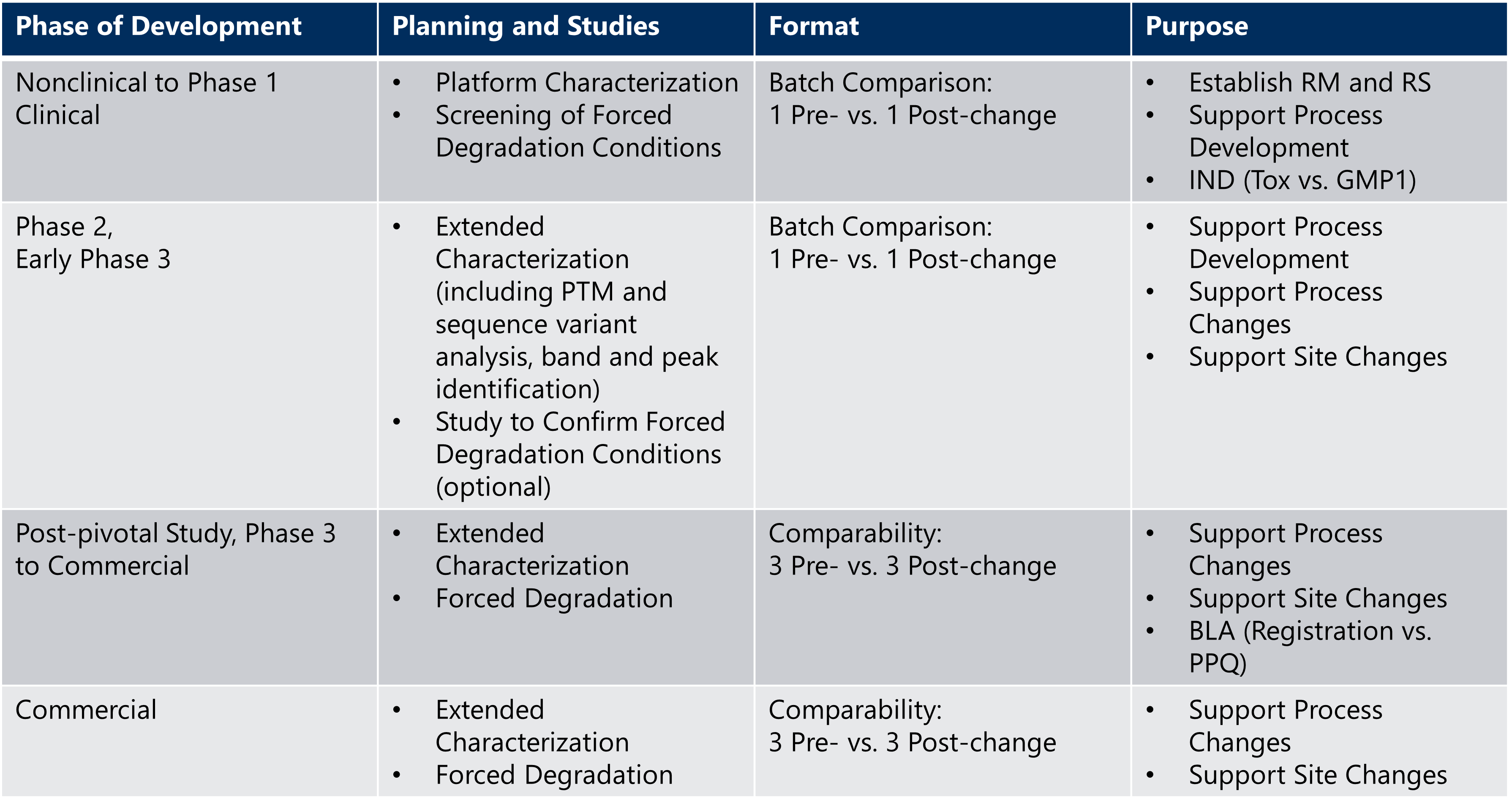
BLA = Biological License Application; GMP1 = first lot manufactured under good manufacturing practices; IND = Initial New Drug Application; PTM = post translational modifications; PPQ = process performance qualification lots; RM = reference material; RS = reference standard.
For early phase development, when representative batches are limited and the critical quality attributes may not be fully established, it is acceptable to use single batches of pre- and post-change material to establish the biophysical characteristics using platform methods (Ambrogelly et al, 2018).
Screening forced degradation conditions early in development can help the analysts gain further understanding of the molecule, inform analytical test method limits, create post-translational modification (PTM) or charge variant identification strategies, and prepare for formal forced degradation studies.
As development continues into Phase 3, extended characterization and forced degradation studies will increase in complexity to include more molecule-specific methods and head-to-head testing of multiple pre- and post-change batches (leading to the gold standard format: 3 pre-change vs. 3 post-change).
Extended characterization of the drug substance can demonstrate an orthogonal approach and more thorough understanding of the unique qualities of the monoclonal antibody, as shown in the example testing panel in Table 2 below.
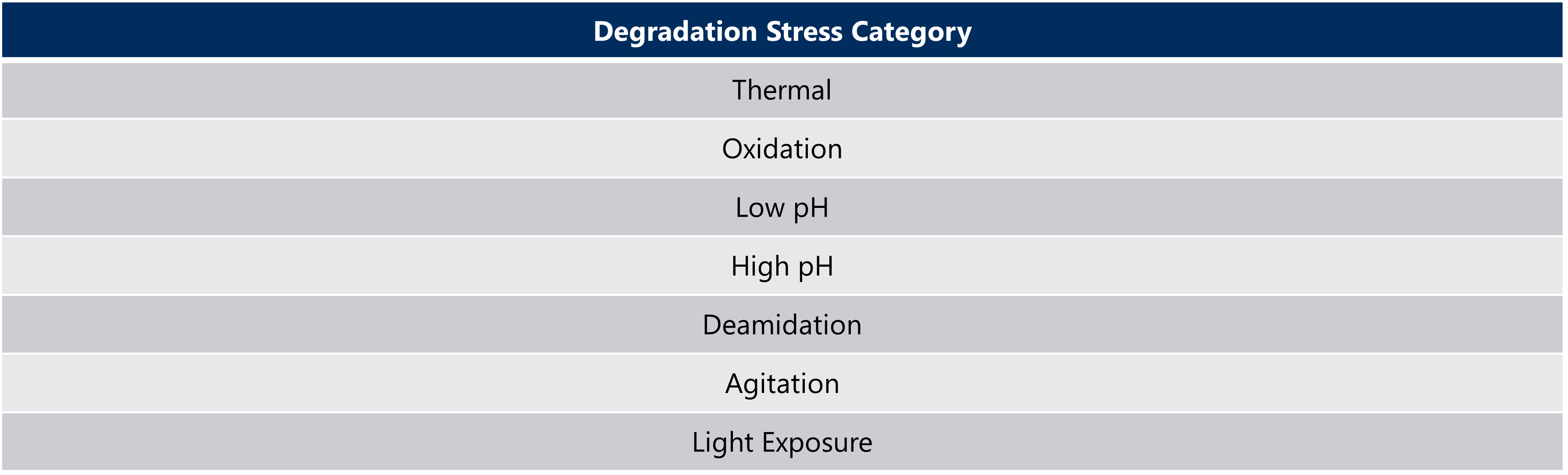
Once the stress conditions, as seen in Table 3, have been selected and optimized, forced degradation of the pre- and post-change batches can unveil the degradation pathways that have previously not been observed in the results of real-time or accelerated stability studies. Proper planning and execution of this pressure-test will demonstrate the quality alignment between the two processes through the analysis of trendline slopes, bands, and peak patterns (Nowak et al, 2017).
Unexpected results from extended characterization and forced degradation studies can open test methods and/or processes to intense scrutiny and further questions. However, facing those challenges early can save time and energy by enabling internal teams to identify and mitigate risks before initiating the expensive, later phases of development.
Learning and communicating as much as possible about the molecular characterization and degradation patterns, especially if unexpected results emerge, can help teams to prepare for regulatory scrutiny and information requests, should they arise down the line. Overall, a strong comparability package for biologics can clear the road to drug approval.
Table 2: Example of Extended Characterization Testing for Monoclonal Antibodies
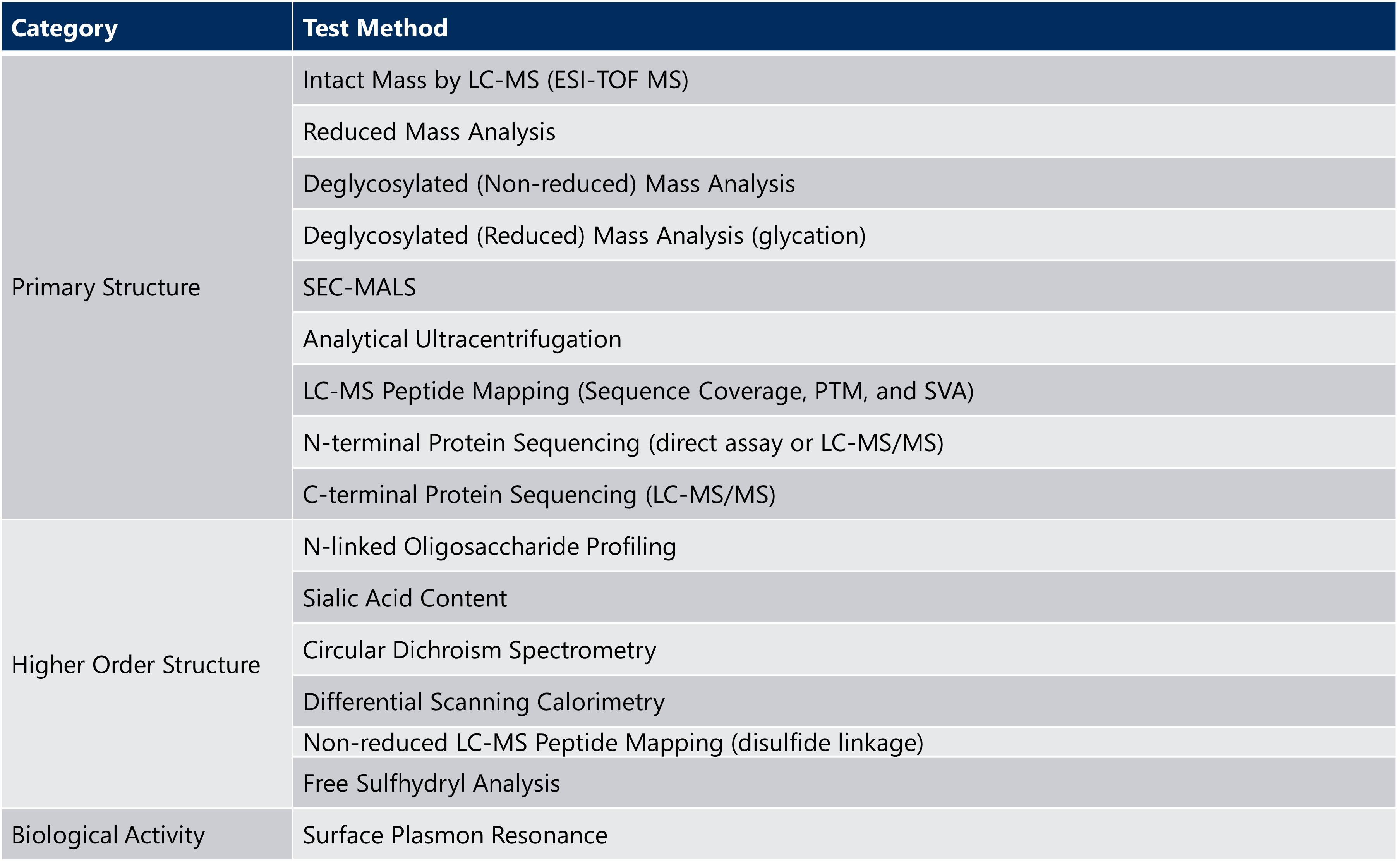
ESI-TOF MS= electrospray time-of-flight mass spectrometry; LC-MS = liquid chromatography-mass spectrometry; SEC-MALS = size exclusion chromatography-multi-angle light scattering; SVA = sequence variant analysis
Table 3: Types of Forced Degradation Stress
Considerations and challenges of comparability studies for biologics
Addressing potentially problematic items in advance of the study by using a well-planned clinical study protocol can prevent difficult discussions during result analysis and reporting. For instance, reiterating that characterization and forced degradation studies are non-GMP, or explaining the lot selection strategy should be captured in the protocols so that reviewers from different backgrounds can understand the rationale.
Extended characterization analytical methods are critical in demonstrating comparability, as they provide a finer level of detail that is orthogonal to release methods, especially for critical quality attributes. Pre-defining both the quantitative and qualitative acceptance criteria for extended characterization methods in the comparability study protocol can alleviate pressure to interpret oftentimes complicated, subjective results as “comparable” or “not comparable.”
Lot selection for comparability studies in biologics is essential as batches should be representative of the pre- and post-change processes or sites. The pre- and post-change batches should be manufactured as close together as possible to avoid natural age-related differences, which could convolute the results.
It is recommended to use the latest available batches that have passed release criteria to avoid even the appearance of “cherry-picking.” Further, the selection strategy should be defined in the comparability protocol or study plan before testing begins.
For forced degradation studies, it is important to note in the comparability study protocol that treated samples are not expected to meet release acceptance criteria as the treatment conditions are outside of typical process ranges. Including a summary of previously tested conditions can simplify future studies by eliminating the need to re-test those conditions that either didn’t show sufficient degradation or by highlighting risks that may or may not be mitigated within the manufacturing process.
The discontinuation of photostability testing, for instance, can be justified if it is observed that the container closure system can protect the monoclonal antibody in the final drug product formulation. However, careful consideration of claims is needed as the drug substance may not necessarily be protected from light during lengthy purification steps.
Proper planning and demonstrating control
While regulatory authorities don’t expect all attributes of a biologic to be identical throughout the product lifecycle, it is the responsibility of the manufacturer to demonstrate that control is maintained in each version of the process, so delivery of high-quality product is ensured.
Additionally, it is expected that the molecular properties of the protein are well characterized and understood so that observed differences between processes can be explained. Good planning of comparability studies can provide that scientific foundation, supporting the complex details needed to maintain a high-quality biologic throughout many process and site changes, and helping to establish the company as a trusted leader in the pharmaceutical industry.
Ultimately, a strong comparability study package for biologics will leave regulators with a sense of confidence in the product and in the company, paving the way for new drug approvals and future endeavors.
If you’re interested in talking with a CMC writing expert, Please click here to connect with the right resource to respond.
Authored by: Christine Nowak, Senior Medical Writer, Regulatory and Medical Writing.





