New Investigator Updates need to be made in the clinical study protocol and kept up to date to with the FDA. And, with more than 32,000 principal investigators globally, according to Applied Clinical Trials, these updates can occur more often than many clinical trial sponsors would like.
For instance, when a new investigator is added to a clinical study or their information has changed from a previously submitted protocol, the clinical trial sponsor of an IND application is expected to submit a protocol amendment.
This is called a New Investigator Update (a protocol amendment to Appendix 16.1.4).
Options for new investigator updates
There are different options that a clinical trial sponsor might explore to keep their clinical study protocols current and correct. Sometimes it is more reasonable to provide the clinical trial sponsor with an update only when a change is made.
This option is best when there are limited sites and updates, such as principal investigator (PI) changes, secondary investigator (SI) changes, and site terminations, are very infrequent. That being said, many clinical trials involve dozens of sites which could generate a steady flow of needed updates.
In this case a rolling monthly New Investigator Update may be ideal.
There can also be unique complications when navigating New Investigator Updates. Non—US clinical sites may refuse to sign the Form 1572, which is pivotal documentation for this type of update. In 2018, the FDA implemented a process for waiving the 1572 signature, but there are specific nuances involved in this process, including distinct waivers and information requirements.
How often should New Investigator Updates be done?
If a continuous periodic update is performed, it should be done on a monthly basis.
There are two reasons for this. This type of update takes into account:
- Adding a new investigator, and
- Updating information related to the investigator’s site, which include investigators.
The CFR 312.30(c) explains that “A sponsor shall submit a protocol amendment when a new investigator is added to carry out a previously submitted protocol” and goes on to explicitly state that, “The sponsor shall notify FDA of the new investigator within 30 days of the investigator being added.”
The CFR 312.23(a)(6)(iii)(b) explains that “the name and address and a statement of the qualifications (curriculum vitae or other statement of qualifications) of each investigator, and the name of each sub investigator (e.g., research fellow, resident) working under the supervision of the investigator; the name and address of the research facilities to be used; and the name and address of each reviewing Institutional Review Board” must be noted in the protocol.
This is a protocol amendment, which are commonly accepted to be updated within 30 days if a change has occurred.
Where is it stored?
This information is stored in module 5.3.5.1 “Study reports and related information of controlled clinical studies pertinent to the claimed indication.” This is a sub-category of 5.3.5 “Reports of efficacy and safety studies,” under module 5 efficacy.
What else is updated and submitted in conjunction?
Site information updates of ClinicalTrials.gov – PRS can be performed in conjunction with New Investigator Updates.
The clinical trial disclosures department would access ClinicalTrials.gov PRS, navigate to the “Contacts/Locations” page of the study, and edit the principal investigator (PI) and site information with the updated information in the final Table 16.1.4.
Please note that PI name and contact details are not mandatory fields in PRS so it is important to discuss any changes with the clinical trial disclosures department as to what would be appropriate to disclose.
Transfer of obligation updates can also be performed periodically in conjunction with New Investigator Updates.
The transfer of obligation document does not tend to change very often, so if the clinical trial sponsor wishes to regularly update the FDA, a quarterly submission rather than a monthly submission may be appropriate. The choice to perform periodic submissions may be reliant on how often the clinical sponsor expects the clinical research organization (CRO) obligations to change.
What does a New Investigator Update look like?
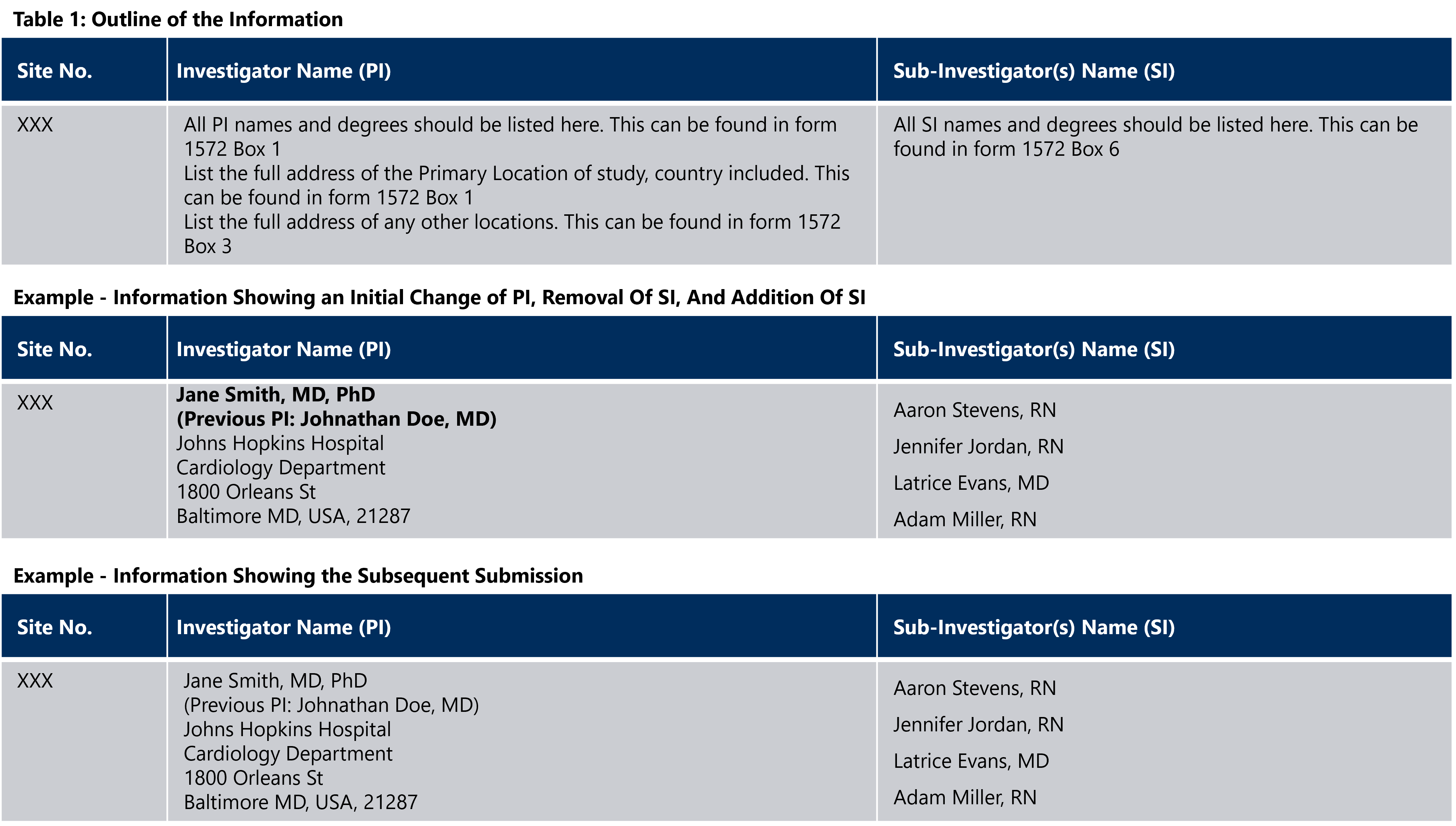
There is no standardized convention for a New Investigator Update. However, the following method could be used to clearly distinguish between newly updated and previous PI site information:
- New information is bolded and oriented at the top of the list
- Terminated site information is marked with a strikethrough and bolded
- Previous PI information that is being replaced with new information is marked with the word “previous” in parentheses, bolded, and oriented below the new information
- Previous site information that is being replaced with new information is bolded and marked with a strikethrough
- Upon the second submission, all earlier bolded writing will be unbolded and previous SI information will be moved to the bottom of its column
- Sponsor CO will ensure that the table information is competed, the column displaying the “Site No.” is completed, and sub-investigator and investigator columns contain name, degree information, and site address information.
The following is an example of what a New Investigator Update may look like:
What documents are sent to the FDA?
Once ready, the following documents may all be submitted together to the FDA:
- updated List and Description of Investigator’s table,
- signed 1571 Form,
- a cover letter briefly describing what is being submitted, and
- if desired, the periodic Transfer of Obligations (ToO).
Obtaining 1572 Waivers for Foreign sites
As of 2021, 1572 waivers fall into two distinct categories: IRB waivers and signature waivers.
For IRB waivers, countries outside the United States may have different requirements for their ethical review committees that may not meet all US institutional review board (IRB) and 1572 requirements. IRB waivers should be requested when the clinical trial sponsor plans to conduct a clinical study under an IND at a foreign clinical site that does not utilize a US IRB, but instead an ethics committee or equivalent organization that complies with ICH E6 GCP requirements.
Once an IRB waiver is granted, the sponsor and investigator are responsible for ensuring that the clinical study conduct is in accordance with the terms of the waiver.
Due to growing pressure from EU countries, the FDA released draft guidance in 2021 that allows for 1572 signature waivers. These waivers are intended for countries where an investigator cannot or will not sign the FDA 1572 form due to local laws, regulations, or customs. To obtain a signature waiver, an alternative course of action will need to be established and approved by the FDA that satisfies the purpose and requirements of the 1572.
This new signature waiver option gives sponsors an additional choice on how to conduct global IND studies and classify ex-US sites in compliance with 21.CRF.312.
The decisions on how to classify ex-US sites, which waivers are applicable, and when to apply for them can be difficult and requirements associated with these waivers can be intimidating. With the EUCTR and ACT EU, the harmonization of clinical trial regulations across EU member states will continue, requiring a flexible, informed approach to meeting the requirements of an FDA IND, form 1572, and waiver requirements.
How MMS approaches New Investigator Updates
To methodically move forward, MMS recommends considering these five checklist items:
- Discuss a monthly process for reviewing changes in site information, so that Appendix 16.1.4 can be properly updated
- Discuss with the transparency team what information should be updated on the ClinicalTrials.gov PRS and who is responsible for completing the updates
- Discuss the interest in having regular Transfer of Obligations updates
- To aid with this process, an intermediary record known as a Protocol Site Listing can be used to help to keep track of site modifications from the 1572 documents to Appendix 16.1.4
- Determination applicable waivers and how to classify ex-US sites should be discussed
It is recommended that the above aspects are discussed early on, preferably during the kick-off-meeting alongside other intricacies of the process including source documents, deliverables, timelines, and document signatories.
For additional information or questions regarding New Investigator Updates, Please click here to connect with the right resource to respond.
Authored by:
Aaron Pyle, Regulatory Affairs Associate , Regulatory Affairs.
Ritchie Patton, Manager, Regulatory Strategy, Regulatory Affairs

