A room full of statisticians and clinical programmers were floored to hear that regulatory operations experts were working with nearly 500,000 pages of text in a recent New Drug Application (NDA). This is how Mary Anne Potok, Technical Manager, Regulatory Operations, MMS, described the weight of one NDA submission to a full house during the PhUSE single day event at Vertex Pharmaceuticals in Boston, Mass. on April 26, 2018.
Handing off data and submission files from clinical programming and biostatistics functions to the regulatory operations team can be challenging and often rate-limiting to timelines if not handled properly. Four common issues in this process that Potok referenced include:
- Issues with source files: Clinical Study Reports (CSRs) from different sources often over a 10-year or sometimes longer span, incomplete legacy and raw data, including datasets without STUDYID and missing define files, and preclinical data only available in PDF format are all major contributors
- Ineligible receivers: With both groups working in separate validated systems, issues may arise, such as: programming outputs that may have been stored on a locally hosted server, eCTD submissions are published in a cloud system, and access limited by role
- Interference: This includes last-minute changes and requests for additional tables, requests for last minute urgent unplanned outputs, physical location and time zone differences, working in siloes, and overlapping priorities
- Data package conversion: Clinical programming and statistics deliverables may be presented in unacceptable file formats for the eCTD (correct file formats can be viewed in the chart below)
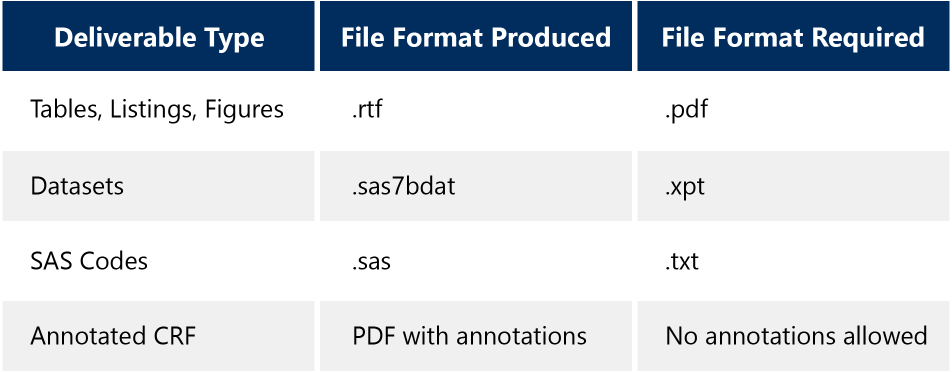
In a recent NDA for example, data packages made up 41 percent of files and one third of the eCTD folder, and Potok suggested “With the goal of preparing and submitting a NDA to the FDA under compressed timelines, the programming, statistics, and regulatory functions need to act as one.”
Quite often sponsor teams work on an NDA every three to five years or after longer durations, their experience may need to be refreshed. To move forward, and resolve these issues, Potok laid a clear path of three clear steps: setting a communications plan, implementing cross-functional training, and engaging in advanced planning.In a recent NDA for example, data packages made up 41 percent of files and one third of the eCTD folder, and Potok suggested “With the goal of preparing and submitting a NDA to the FDA under compressed timelines, the clinical programming, statistics, and regulatory functions need to act as one.”
Set a communications plan
A detailed communication plan ensures each team member is on track to ensure a successful submission to the agency. The plan should include weekly meetings between programming, statistics, and regulatory operations. Early communication leads to easy alignment on items, including table numbering strategies that facilitate clear hyperlinking to tables, and welcomes regular discussion of upcoming deliverables, shifting priorities, and potential impact to timelines.
If working with a trusted partner, regulatory operations should be included on meetings between the data team and sponsor, including a process that keeps the regulatory lead copied on all notes related to data package deliverables.
Prior to submission kickoff, the Study Data Standardization Plan should be reviewed to understand data requirements, and all agency interactions should be shared with all team members supporting the submission.
Host cross-functional training and support
Once communication is set, training is the next big focus area. Clinical programming, biostatistics, and regulatory teams should be trained together on FDA updates for data requirements. This may be through business and industry webinars, internal processes, and joint reviews of FDA guidances for shared understanding and interpretation, such as the Study Data Technical Conformance Guide.
Cross-functional training allows for key stakeholders to be granted read-only access in each other department’s systems. When implementing this training herself with several sponsors and teams, Potok added that workflow is generally not disrupted and it ensures that all team members have access to the same time-saving tools.
Engage in advanced planning
Planning in advance may not seem like anything new, but pre-formatting reviewer’s guide templates and SAP templates are tasks that can save valuable hours during the final publishing crunch. On the data side, it can save regulatory operations time if a list of datasets and relative size of files is provided by programming in advance of the files being complete. This allows regulatory to prepare placeholders for the datasets in advance.
Finally, teams should review deliverable timelines over critical periods to carefully choreograph the transition of datasets during slower document publishing times.
Potok has witnessed teams work outside of their comfort zones in these three steps to achieve successful and refined NDA submissions. Not only that, but it allows members of each functional line to serve on a joint submission team, promoting collaboration. Potok concluded, “Once you reach across the aisle to programming and statistics, it’s easy to realize how alike the processes are, even though our deliverables are vastly different. Working concurrently, not consecutively, delivers consistent victories.





