The CDISC Standard for the Exchange of Nonclinical Data (SEND) requirements for US Investigation New Drug Applications (INDs), New Drug Applications (NDAs), and Biologics License Applications (BLAs) requirements, designed to improve the ease of nonclinical data review at the FDA, should be considered in the context of improved efficiency.
In planning a study startup or filing an application for approval, time spent understanding SEND invariably saves time downstream and results in a more predictable submission.
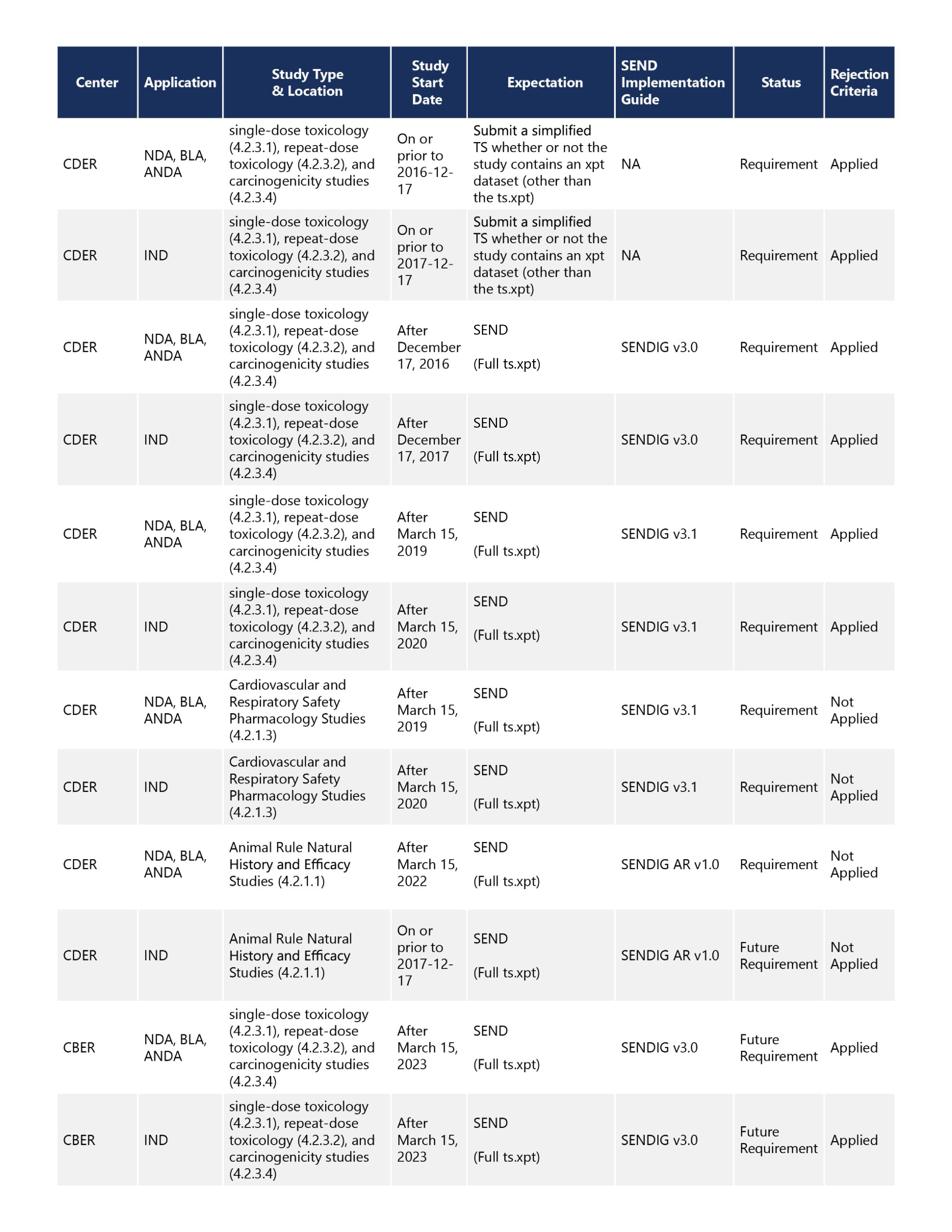
The relevant study start date cutoffs and expectations for specific study types requiring SEND datasets differ by center and application type, as shown in Table 1.
What Are the Requirements for SEND data?
While both CBER and the CDER strongly encourage IND Sponsors and NDA applicants to consider implementing and using these study data standards as early as possible in the product development life cycle, SEND data is currently only required for nonclinical reports filed to CDER.
Depending on the study start date, Sponsors may be required to submit a simplified Trial Summary (TS) file, full TS file, or full SEND datasets. TS files contain information about each study used by the eCTD validator to determine if SEND data is needed. Thus, at minimum, some form of TS file is required for all eCTD submissions to CDER shown below.
Sponsors filing applications to CDER can check their study type against the study start date to determine which files are required and whether failure to include a simplified TS file, full TS file, or full SEND data will result in a technical rejection.
A submission that receives a technical rejection fails eCTD validation. Thus, rejection occurs before the submission goes through the gateway, a potentially costly error for which there may be limited recourse beyond adding the appropriate file to the submission.
Sponsors with plans to submit NDAs, BLAs, and Abbreviated New Drug Applications (ANDA) to CDER should note that the study start date requirements for submitting SEND data to these applications are generally a year earlier than the IND requirements. Thus, it is possible that Sponsors would have to file SEND to an NDA/BLA/NDA even if not required for the IND.
To ensure sufficient preparation time, Sponsors of biologics should note that requirements for CBER, which will go into effect for single-dose toxicology, repeat-dose toxicology, and carcinogenicity studies, started after March 15, 2023. Therefore, understanding the requirements will be essential to avoid rejection for INDs starting as early as the second quarter of 2023.
Sponsors should also note that SEND datasets are typically required for both interim and final reports. It’s important to consider the overarching rationale for SEND: to increase the efficiency with which nonclinical data required for specific regulatory decisions can be evaluated. Consistent with this objective, the SEND data should be filed concurrently with the decision that is being made.
Tips for Working with SEND Data
Because these rules are relatively new and variable, it is recommended that Sponsors use a Study Data Standardization Plan (SDSP) during development to communicate the intent to submit SEND datasets or to explain further the intended use of simplified or full TS files.
For INDs, an SDSP should be submitted in the General Investigational Plan of the Initial IND. For NDAs and BLAs, an SDSP should be provided with pre-NDA and pre-BLA meetings (appended to BD or cross-referenced).
If you have any questions about SEND, please email info@mmsholdings.com to connected to an expert.
Find out more about MMS regulatory operations on our website.
By: Ben Kaspar, Director of Regulatory Affairs, Swathi Pandhiti, Associate Director of Regulatory Operations





